Recruitment of participants
Participants were selected from a pool of 220 participants who had previously taken part in a prediabetes screening study. The recruitment took place at the three largest PHC clinics in North Iceland [21, 22]. The power estimates for sample size were derived using a population size of 21,000 individuals aged 18–75 years living in the research area in the year 2019, as reported by Statistics Iceland [23]. In the calculation of sample size to establish eligibility for allocation in the RCT the confidence interval (CI) level was set at 85% with a margin of error of 5%. A minimum sample size of 206 participants in the screening study was needed to conduct this RCT, and at least 30 in each group at time 1 measurement. Although a larger sample, at 378 would be good and provided CI level of 95% with a 5% margin of error, this was not a viable sample size to recruit, given the population size and dispersion in the sparsely populated research area at the time of the Covid-19 pandemic.
Individuals between the ages of 18 and 75 who resided in the service area of the participating PHC clinics, were eligible for participation. Participants were required to not have a diagnosis of T2D and had fluent proficiency in either Icelandic or English. The enrolment criteria were based on achieving a score of ≥ 9 points on the Finnish diabetes risk score scale (FINDRISC), in addition either or both, BMI ≥ 30 kg/m[2] and/or HbA1c of ≥ 40 mmol/mol. The FINDRISC scoring scale estimates the risk of developing T2D within the next 10 years, using eight questions and assigns a score ranging from 0 to 26 points, with increasing risk with higher scores [21, 24].
Allocation into intervention and control groups
The 81 eligible participants, who satisfied the enrolment criteria, were allocated a numerical identity by the first author (EA). These identifiers were then placed in separate jars based on age (below or over 50 years) and gender. The second author (AKS) picked a pair of numbers from each of the two jars. The first number in each pair belonged to the intervention group, while the second number belonged to the control group. This procedure persisted until all the numbers were selected. Subsequently, the author EA reached out to the participants to arrange a single measurement session, either at the nearest PHC station or at the research centre. The allocation ratio was in favour of the intervention, with a ratio of 1.08:1.00. This preference was taken for any potential dropouts throughout the intervention. Of the 81 eligible 64 participated in time 1 measurement or 79%.
The intervention group
The GSD counselling approach intervention had not been previously employed in Iceland. Hence, the six nurses, two assigned to each PHC clinic who were implementing the intervention, attended a one-day workshop on GSD in March 2020 at the research centre, facilitated by the last-author BCHK. The seminar included ongoing assessment and feedback. Prior to starting the intervention, an online meeting was conducted with the six nurses and BCHK to enhance their proficiency in providing GSD counselling. The participants were given a notebook to record their thoughts and comments in, as well as a sheet with prompts and questions for reflection, mirroring, and active listening during the GSD intervention. During the intervention period, the nurses were contacted by EA over an encrypted Microsoft Teams© channel and phone. They were asked if they had any questions regarding the delivery of GSD or if they needed any assistance. In addition, the nurses had the option to communicate with EA or BCHK by phone or through the Teams channel for any inquiries.
The GSD intervention started in late December 2021 and finished in April 2022. The duration of each GSD counselling section (intervention) was approximately 60 min, with 4–6 weeks intervals in total of 12–16 weeks. Between counselling sections, the participants were instructed to complete the GSD reflection sheets that were given to them during their first GSD counselling with the nurse [19]. The sixteen GSD reflection sheets handed to participants had previously been used in a study with individuals at risk of developing T2D and with manifest T2D in Norway. The sheets were then translated from Norwegian to Icelandic for the purpose of this research. During each consultation session, the participants engaged in discussions with the nurse on their notes on the reflection sheets, while also establishing objectives for their health promotion. Following the measurements at time 1 (baseline), the intervention group was notified by EA that a nurse from the nearest PHC would contact them for three GSD consultations, spanning a duration of three to four months.
The control group
The control group were at time 1, following the measurements, given a booklet from the Icelandic Directorate of Health [25] on healthy diet choices, and informed that the next part of the research would be repeated measurements that would take place in 6 and 9 months from time 1 measurement.
Instruments, and biological measurement
Primary outcome, the Icelandic Heart Association coronary heart disease risk calculator
For more than 50 years, the Icelandic Heart Association has gathered data from a comprehensive health survey, which includes statistics on the prevalence and death rates of coronary heart disorders (CHD) [26]. An outcome of this scientific research is the development of an open access heart disease risk calculator (ICE-HEART), which evaluates an individual’s probability of CHD over the course of the following decade [27]. The ICE-HEART is comparable to the SCORE risk calculator developed by the European Society of Cardiology [27]. The ICE-HEART CHD risk calculator ( provides an estimate of an individual’s probability, expressed as a percentage, of developing CHD during the following 10 years. This estimate is compared to the average probability of CHD for individuals of the same gender (male/female) and age [27].
The primary outcome of this RCT was set as a change in the relative risk (RR) of CHD, when compared to individuals of the same age and gender, both within and between the intervention and control groups. This was assessed using the open access online ICE-HEART CHD risk calculator [27]. The calculator incorporates measures of age (within the range of 35–75 years), height (ranging from 150–200 cm), weight (between 45–120 kg), systolic blood pressure (ranging from 100–200 mmHg), total cholesterol (CHOL) levels (ranging from 4–10 mmol/L), high density lipoprotein (HDL) levels (ranging from 0.5–2.5 mmol/L), and triglycerides (TRG) levels (ranging from 0.5–4.5 mmol/L). Furthermore, the variables required for the assessment of CHD risk include the levels of physical activity, smoking patterns, presence of diabetes, and family history of CHD [27].
The height was recorded using a portable measuring tape, rounded to the nearest 0.1 cm, while the weight was measured on a digital scale, rounded to the nearest 100 g. The measurements were taken when the person was wearing light clothes [28].
The cholesterol (CHOL), high-density lipoprotein (HDL), and triglycerides (TRG) levels were measured using a Mission® cholesterol meter. In addition, ICE-HEART provides computed tests for low-density lipoprotein (LDL) and the ratio of total cholesterol to high-density lipoprotein (CHOL/HDL).
Participants’ blood pressure (BP) was measured at the end of each section after they had been seated for a duration of 10 min. Utilizing the Medisana® upper arm meter for measuring [29]. The treatment threshold for systolic pressure is commonly set as ≤ 140 mm/Hg and for diastolic pressure as < 90 mmHg [30]. Research indicates that elevated BP raises the likelihood of developing CVD [31, 32].
Secondary outcomes and definition of T2D and prediabetes level
The Body Mass Index (BMI) ≥ 30 kg/m[2] was one of the inclusion criteria, calculated using height and weight, reported in kg/m2. A BMI ≥ 25 kg/m2 indicates overweight and a BMI ≥ 30 kg/m2 indicates obesity [32]. The measurements of waist circumference, taken 2 cm above the navel, and hip circumference at the widest point, as well as height in centimetres, were recorded using a measuring tape with a capacity of either 1.5 or 3 m. The accuracy of the measuring tapes was frequently assessed. The waist-to-height ratio WHtR was computed by dividing the waist measurement in cm by the height measurement in cm. The WHtR is not influenced by gender or ethnicity. Previous study suggests that it may be a more accurate predictor of diabetes risk compared to BMI. A WHtR ratio of less than 0.5 indicates no increased overall health risk, while a ratio between 0.5 and 0.6 indicates an elevated risk. A WHtR ratio of 0.6 or above indicates a very high overall health risk [31, 33, 34]. Previous studies have suggested that the WHtR is a more effective screening tool than the Waist-to-Hip ratio (WHR) for identifying individuals at risk of cardiovascular disease (CVD) and T2D. This is because an elevated WHtR is a stronger indicator of both conditions compared to an elevated WHR [22, 31, 34,35,36].
WHO has approved the HbA1c level as a diagnostic test of T2D [37]. We employed the DCA Vantage® system (manufactured by Siemens Medical Solutions Diagnostics Europe Limited, based in Dublin, Ireland) to analyse capillary blood samples. The American Diabetes Association classifies HbA1c levels between 39 and 47 mmol/mol (5.7 – 6.4%) as prediabetes, whereas levels of 48 mmol/mol (6.5%) or more are considered indicative of T2D [38].
Fasting glucose (FBG) was measured using a capillary blood sample and the OneTouch® Verio Flex blood glucose meter. The latest advice for diagnostic purposes for T2D is to utilize plasma or fasting plasma glucose [39]. The objective was to conduct a screening for alterations in blood glucose levels during the duration of the RCT. Normal glucose level is defined as being less than 5.5 mmol/l (< 100 mg/dl). Prediabetes is classified as having a glucose level between 5.5–6.9 mmol/l (100–125 mg/dl), whereas diabetes is diagnosed when the glucose level exceeds 7 mmol/l (≥ 126 mg/dl) [40].
Participants in addition answered questions regarding background characteristics, and the FINDRISC tool to record changes over the RCT time [41]. FINDRISC is a questionnaire consisting of eight questions. Each question is scored on a scale of 0 to 26 points. A higher score implies a higher chance of developing T2D in the following 10 years. The FINDRISC has been authorized as a diagnostic test for assessing the risk of developing T2D [42].
Data collection
Baseline measurements were collected from the end of October 2021 to the middle of December 2021, and the next measurement in late spring 2022, and the last in late autumn 2022. Figure 1 displays a visual representation of the measurement timeline. Each participant was given a document with the current biological measures as well as one-time prior measurement at each session. Consequently, they were able to see and compare the differences between the present measurement and a previous measurement.
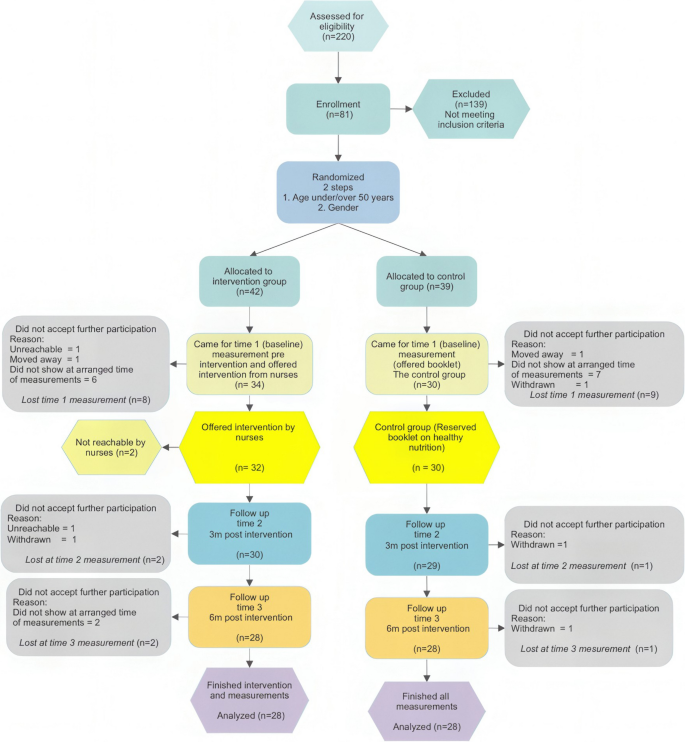
Flow Chart of eligible participants in the intervention
To reduce the number of dropouts, those who did not appear at the scheduled time for measurements were given the opportunity to reschedule their appointment at least two more times before being classified as dropouts. Researcher EA personally interacted with and performed all the measurements on every participant.
Statistical analysis
The ICE-HEART calculator available online at was used to calculate predicted CHD risk for each participant. The ICE-HEART risk calculator includes defined lower and upper limits of values for the variables. The calculator automatically adjusted variables to the highest/lowest applicable score if a participant’s value went outside the range of the ICE-HEART variables. The ICE-HEART calculator provides an individual’s estimated CHD risk, in the next 10 years, in percentages as well as the average risk for the same age and gender. To be able to compare the risk of participants of different ages and genders, the individual risk of each person was divided by the risk of the ICE-HEART same age and gender risk. This enabled calculation of mean CHD risk of each group of participants in comparison with the mean CHD risk of the ICE-HEART cohort. The outcomes were reported as the ratio of the risk for each participant divided by risk of an individual of same age and gender, according to the ICE-HEART risk calculator. Calculations for each participant were verified twice to avoid any potential input mistakes.
The study participants’ changes in CHD risk were assessed from time 1 to time 3. This allowed for the calculation of the “control event risk” (CER), “experimental event rate” (EER), and subsequently the “absolute risk reduction” (ARR) using the formula CER-EER = ARR. The Number Needed to Treat (NNT) for a favourable result is defined as the outcome of the ARR. The relative risk (RR) was determined by dividing the chance of an adverse outcome in the intervention group by the probability of an unfavourable outcome in the control group. Relative risk reduction (RRR) is a measure that quantifies the extent to which the risk of negative outcomes is lowered by an intervention compared to a control group [43]. The absence of any change in CHD risk between time 1 and time 3 was considered a poor outcome. This was because the goal was to decrease the risk, and reporting no change was seen as a more careful and cautious approach to presenting the results of this RCT. Descriptive statistics were employed to analyse continuous data and calculate means, standard deviations, and ranges. The groups were compared using chi-squared calculations, and changes in risk between measurements were calculated using crosstabs for odds ratio (OR), RR, and NNT calculations. The independent t-test was employed to compare the means of continuous variables between groups.
The General Linear Model of repeated measurements was used to calculate the interaction between and within groups throughout time. Due to the potential sensitivity of small sample sizes to sphericity, a mixed ANOVA, was added in analysing the data [44].
Data was analysed using IBM SPSS Statistics 27 and for mixed ANOVA the R version 4.3.1 (2023–07-16 ucrt), using rstatix, lme4, mixed effect model and lemerTest. Missing data, was if applicable, excluded listwise. Statistically significant difference was set at p ≤ 0.05 (two tailed).
Ethical considerations
The present study was performed in accordance with the Helsinki Declaration and with the approval of the Icelandic National Bioethics Committee (VSN), (VSN-19–080-V1 approved 14/01/2020). All participants received verbal and written information and signed an informed consent form before participating. This study is registered at www.ClinicalTrials.gov (NCT01688359) on 30th December 2020. It is titled ‘Effectiveness of Nurse-coordinated Follow-Up Programme in Primary Care for People at Risk of T2DM’. The CONSORT 2010 criteria are utilized for reporting the randomized trial [45].
link

